Neurovalens' co-founders, Dr Jason McKeown and Dr Paul McGeoch, began their journey by collecting data from a benchtop device that had demonstrated a novel and effective way to help people lose body fat. Their vision was for a product to be used by the widest global demographic and, due to the mechanism of action (stimulating just behind the ear), would mean that the device would have to be wearable. When Neurovalens first engaged with i4 the company's headcount was at 2. As a lean start-up, the company were looking for a firm capable of complementing their medical expertise by providing comprehensive design and development support to take their benchtop stimulator into a mass manufactured wellness product.

Due to the novelty and wearable nature of the product there were a number of challenges facing the design and engineering team. Below are some of the Industrial Design and Mechanical challenges overcome:
Some of the systems and electro-mechanical challenges overcome during the project included:

Comfort is a subjective quality and was identified as a key point of measuring the success of the product's design. It was therefore decided that from the outset we should approach the project from both a 2D sketching/3D CAD angle and physical 3D models/prototypes to gain the best result.
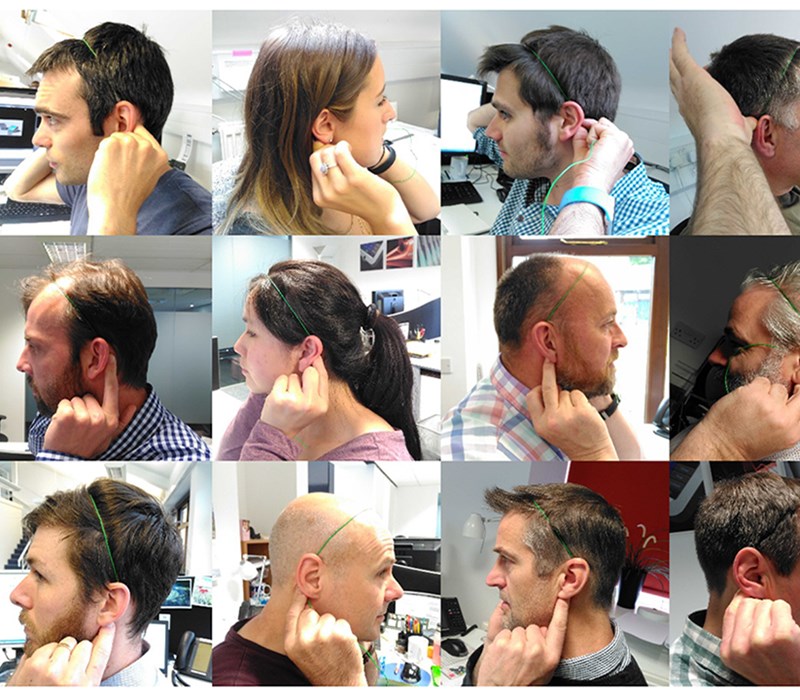
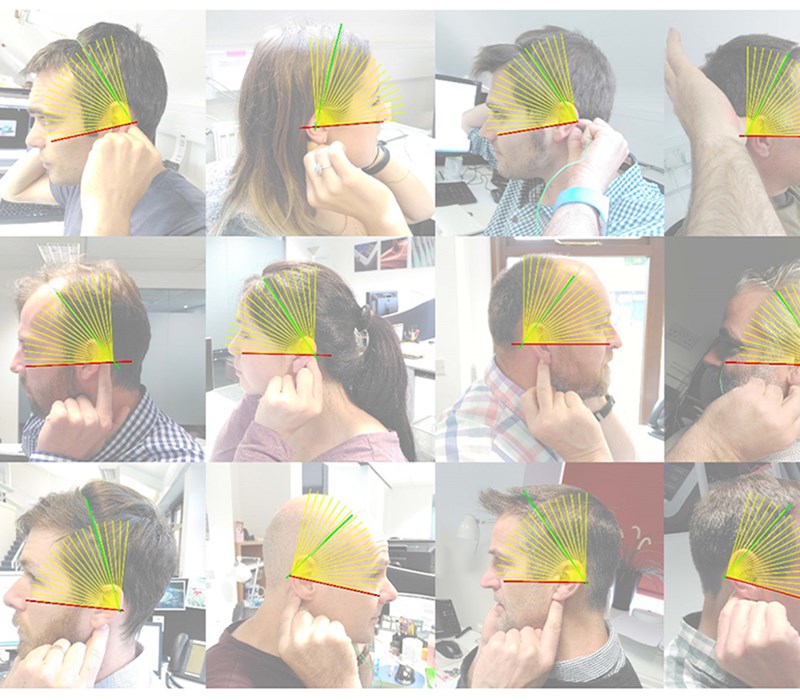
The team determined the 5th percentile to 95th percentile head sizes from ergonomic data we had accrued on our system and backed this up by taking key measurements from the heads of staff in the office. We built a 3D CAD model of these heads and basic test rigs were created to find how people reacted to various fits. This was assessed visually and with questionnaires. See our Human Factors blog for more details.
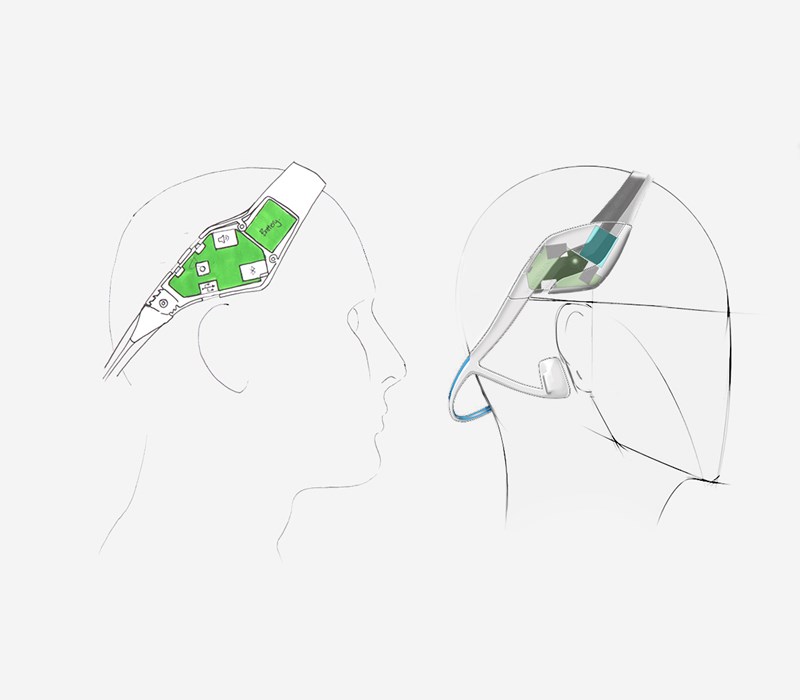

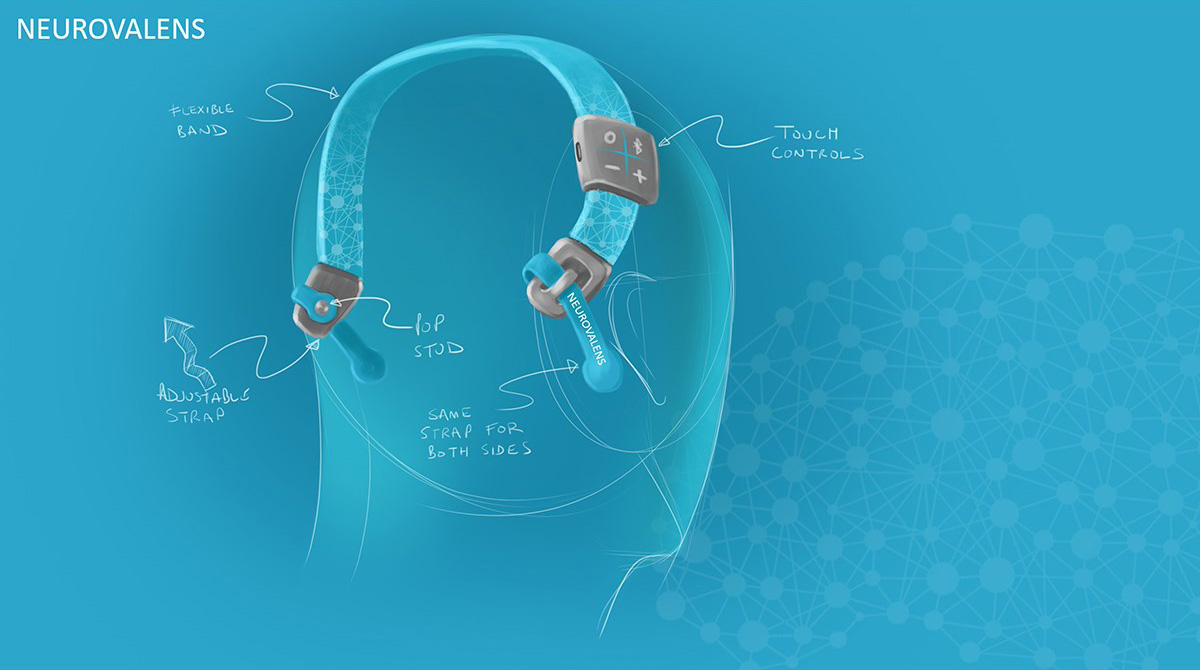
Concurrently with the human factors study the Industrial Design team created some hand sketch ideas of how we could apply the device to the varying head sizes using approximated geometry of the electronics and the ergonomic data we had available to us.
Prototype models, weighted to approximately the correct level, were made and tried by our initial test group. We were able to try various methods of attachment and stimulation delivery to the specific location on the head; taking into account personal preference/hairstyle/ergonomics. We then translated the results into 3D CAD to enable our designers to fine-tune and re-adjust according to our ergonomic market limits.


Neurovalens were specific that the product was to be worn on the head, so as to create a visual link to the part of the body it acted upon. However, the product also had to stand apart from other head wearables such as headphones, to create a unique feel to the product.
The aesthetic also needed to appeal to both women, men and all nationalities. Appealing to all often leads to an unhappy compromise, so we took our physical requirements of ergonomics/contact points/hardware/moulding feasibility and began work.
Initial visual results found that creating a natural visual flow between the above requirements gave a satisfying aesthetic. We took inspiration from the core function of the device which centred around flow. Flow took us visually to organic inspiration in nature, particularly aquatic themes. As this was Neurovalens’ first product, we also had to create a translate-able aesthetic and brand that would allow potential branding, marketing and packaging possibilities.
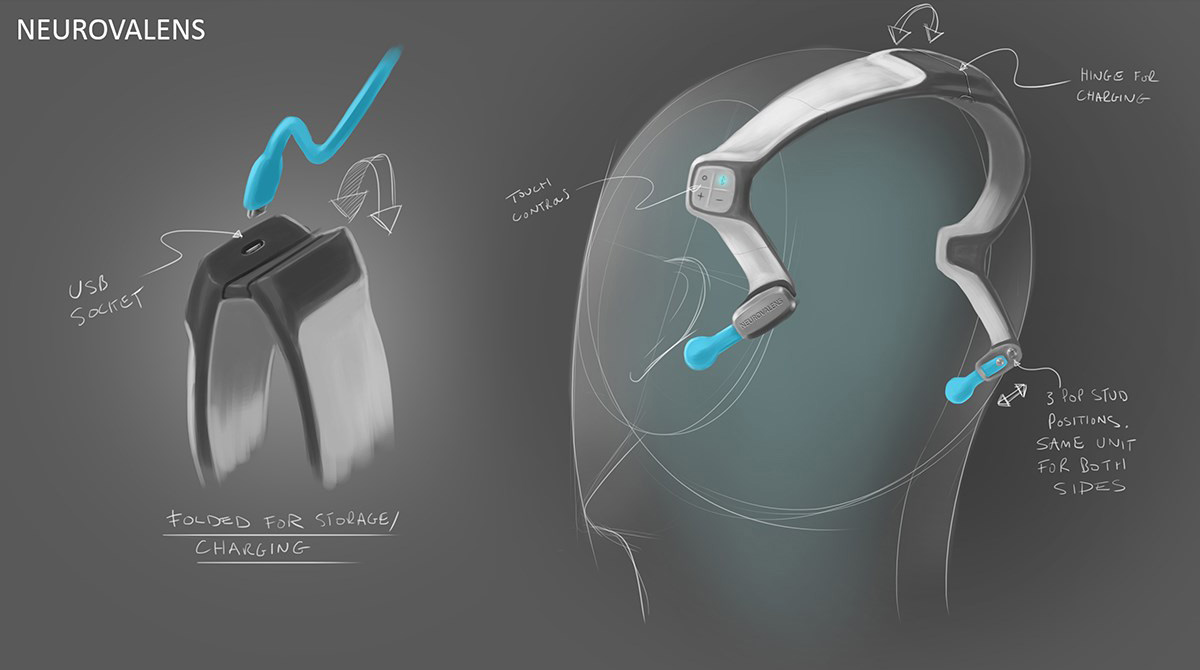
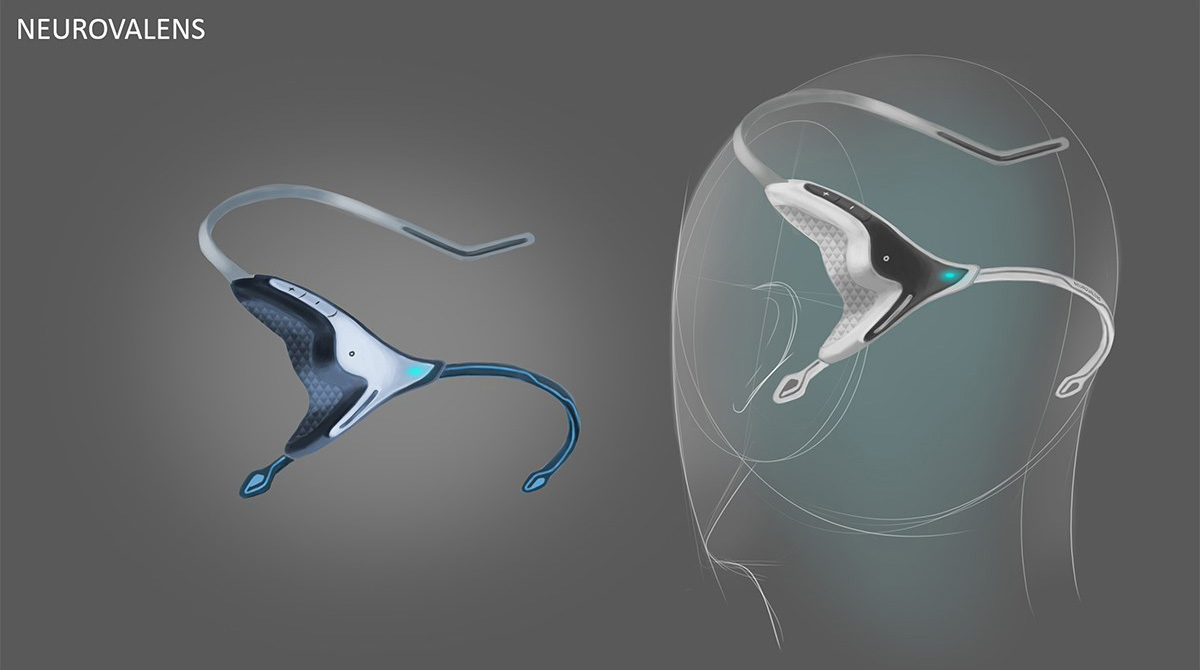
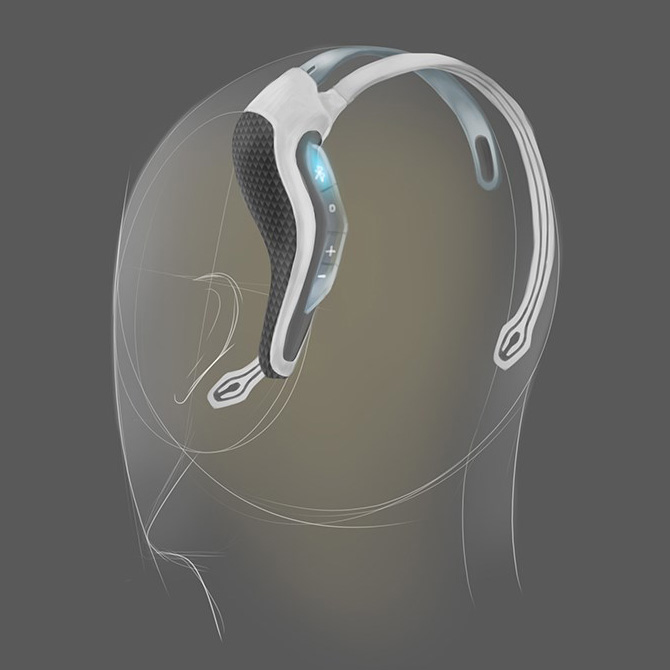
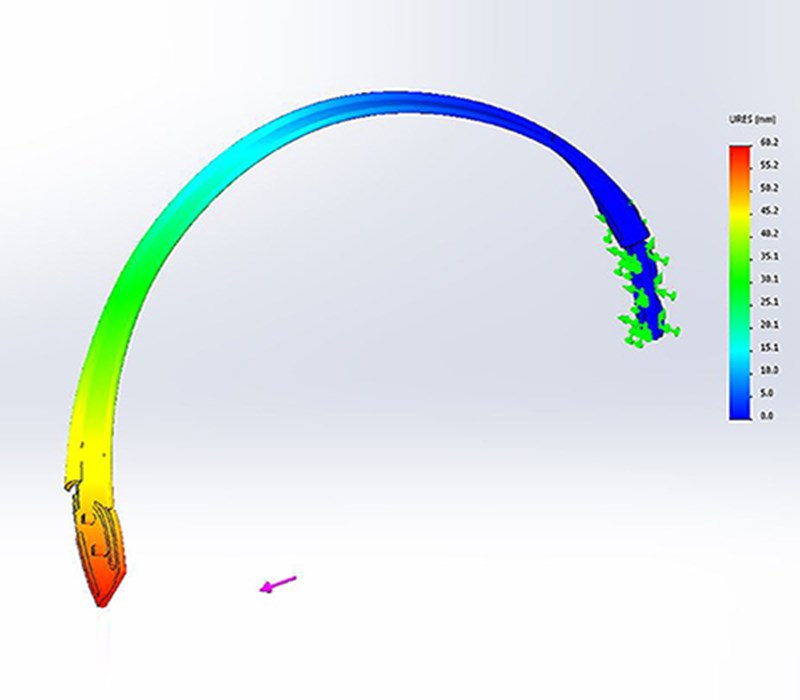
3D printed appearance and functional models were produced at multiple points along the project to verify fit and form. Once the direction on the form was agreed the CAD data was evaluated with FEA to ensure the key components were resilient to the stresses associated with every day use. Once the data was verified a prototype was produced that replicated (as closely as possible) the potential manufacturing materials so a unit could be physically stress tested.
Initially we followed a flexible printed circuit method of transferring stimulation to the head, but following testing we found this to be too awkward and restrictive regarding fit and comfort. We reverted to bespoke cables with industry standard snap studs which are commonly used in other areas of medical equipment.


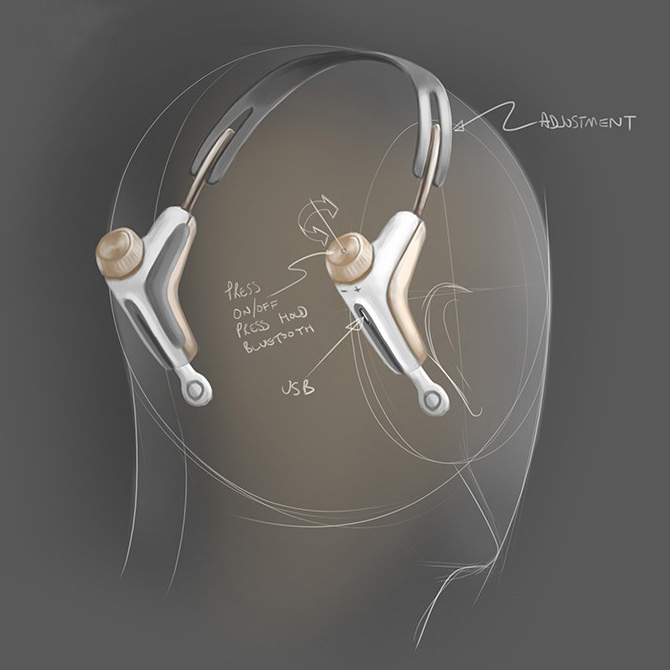
In addition to the physical requirements of the product, we needed to consider how the user could interact with the device and gain feedback. We created a new user interface which could be interpreted whilst out of sight and when used as a standalone device; in addition to when being used in conjunction with a smartphone via the app.
i4pd supplied functional and aesthetic prototypes to Neurovalens' for their exhibition at Arab Health 2017 which afforded the company early market feedback on how the new product would be received by direct customers and large scale buyers.
Strict tooling budgets and timescales restricted the number of parts that the product could be made up from and the materials used. Our design engineers were looking for a combination of stability, toughness, flexibility, appealing surface finish and cost. We considered many approaches, but calculated that the best possible result would be achieved using a variety of materials used each for their specific properties and best fit for purpose. As each part could potentially vary in look and feel, the contact points where they met needed to be treated extremely carefully and treated via detailing to provide a visibly pleasing result.
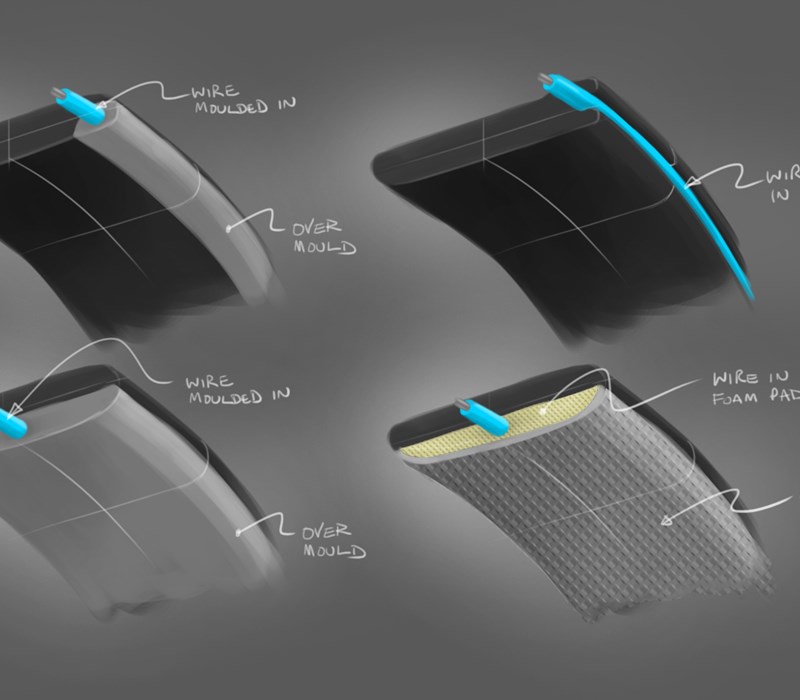
The device also had a requirement to be environmentally responsible at the end of its life, so we needed to allow for product disassembly and component breakdown for recycling streams. We were aware that we needed to create a device that was mechanically fixed together, meaning more engineering detailing on internal surfaces than a “bonded together” fixed device. This in turn led to more design constraints as components would need to be removable.
Throughout the engineering design phase we worked closely with manufacturers to ensure the designs were suitable for mass production. This included working with Neurovalens’ chosen assembly house, Elite Electronics, to ensure the mechanical design was optimised for ease of assembly.
As Neurovalen's development partner i4pd was also relied upon for tooling and manufacturing support after progressing through the architecture and prototyping phases. This included assisting in the tool designs and ensuring that the ABS, Polypropylene, Polycarbonate and TPE rubber parts were produced to the original design intent, and to the best possible standard due to the high end nature of the product.
As mentioned earlier, a number of different materials were used in this product. However, as expected, due to the complex surfaces of the part, over-molding the front cover of the product proved to be biggest challenge due to the shrinkage rate of the over-mold material. With the expertise of the i4 designers and the tooling knowledge of our manufacturing partner, we overcame this obstacle together.
Visit modiushealth.com for more details and where to purchase.


We like to find new, better and efficient ways of doing things. Contact us to discover how i4 Product Design can solve your current design challenge and take your product to the next level.
Copyright © 2024 i4 Product Design Ltd. All rights reserved. | Privacy Policy